The Complete Engineering Guide to Electroplating: Specs, Costs & Common Failures
By The CNMP Expert Team
Machining the part is only half the battle. If you select the wrong finish, your precision component can turn into a rusty paperweight in weeks, or fail catastrophically under load due to hydrogen embrittlement.
Electroplating is the industrial standard for adding “superpowers” to raw metal: corrosion resistance, conductivity, solderability, and wear resistance.
However, the finishing industry is filled with vague terminology. Asking a shop for “Chrome Plating” could get you a shiny car bumper finish or a dull, industrial hard-facing—two completely different processes with vastly different prices.
In this guide, we move beyond the basics. Instead of just listing materials, we categorize these electroplating solutions by their engineering function, helping you balance budget, compliance (RoHS), and performance.

Category 1: Corrosion Protection (Zinc vs. Nickel)
The Battle for Your Budget.
When your primary goal is preventing rust on steel parts, you have two main options.
1. Zinc Plating: The Cost-Effective Standard
This is the “workhorse” of the fastener and bracket industry. It functions through sacrificial protection—the zinc layer corrodes so the steel doesn’t have to.
- Engineering Spec: ASTM B633.
- Cost: Low ($).
- The RoHS Trap: The color of zinc comes from a secondary chromate dip.
- Yellow Zinc: Historically contained Hexavalent Chrome (Cr6+), which is banned in the EU. You must specify “Trivalent Yellow” to ensure your parts are RoHS compliant.
- Clear/Blue Zinc: Almost always RoHS compliant.
2. Nickel Plating: The Premium Alternative
If Zinc is the “economy” option, Nickel is the “luxury” upgrade. It protects by creating a sealed barrier.
- Electrolytic Nickel: Uses current to deposit metal. It is brighter but suffers from uneven distribution (thick corners, thin holes).
- Electroless Nickel (EN): This is the superior choice for precision parts. Because it relies on a chemical reaction rather than electricity, the coating thickness is perfectly uniform (1:1 ratio) on every surface, including deep bores.
- Cost: High ($$-$$$).
Category 2: Wear Resistance (Hard Chrome)
When hardness matters more than looks.
Do not confuse “Hard Chrome” with “Decorative Chrome.”
In industrial electroplating, Hard Chrome (Engineered Chrome) is designed strictly for tribology—reducing friction and resisting wear.
- Hardness: HRC 65-70 (Harder than a file).
- Thickness: Heavy build-up (0.002″ to 0.010″+).
- Appearance: Matte grey. It is not shiny unless mechanically polished afterwards.
- Application: Hydraulic piston rods, mold cavities, and shafts that run against seals.
Engineering Insight: Hard chrome has micro-cracks that hold oil film, making it excellent for sliding contact surfaces.

Category 3: Conductivity & Electronics (Gold, Silver, Tin)
Managing signals and solder.
For electronic components, surface finishing serves a different purpose: ensuring the flow of electricity.
Gold Plating (The Noble Choice)
- Why use it: Gold never oxidizes. It maintains low contact resistance forever.
- Cost Hack: This process is expensive. Use Selective Plating to deposit gold only on the connector pins, while coating the rest of the shell in Nickel.
Silver Plating (The RF King)
- Why use it: Silver has the highest electrical conductivity of any element, even better than gold. It is essential for Radio Frequency (RF) cavities and skin-effect conduction.
- The Downside: It tarnishes (sulfur attack). Always specify an anti-tarnish dip in your drawing notes.
Tin Plating (The Solder Interface)
- Why use it: Excellent solderability.
- The Risk: “Tin Whiskers.” Pure bright tin can grow microscopic metal hairs that short-circuit electronics.
- The Fix: Specify Matte Tin or a Tin-Lead alloy (if RoHS exempt) to inhibit whisker growth during the product lifecycle.
Critical Risks in the Process
Two ways to destroy a perfect part.
1. Hydrogen Embrittlement
This is a silent killer in high-tensile steels (Rockwell C 40+). During the acid cleaning and electroplating phases, hydrogen atoms penetrate the steel lattice. Under stress, these atoms cause the part to snap unexpectedly.
- The Mandatory Protocol: Parts must undergo a Bake Relief process (typically 375°F for 4 hours) immediately after the tank cycle.
- Your Responsibility: You must explicitly state “Bake for Hydrogen Embrittlement Relief per ASTM B850” on your drawing.
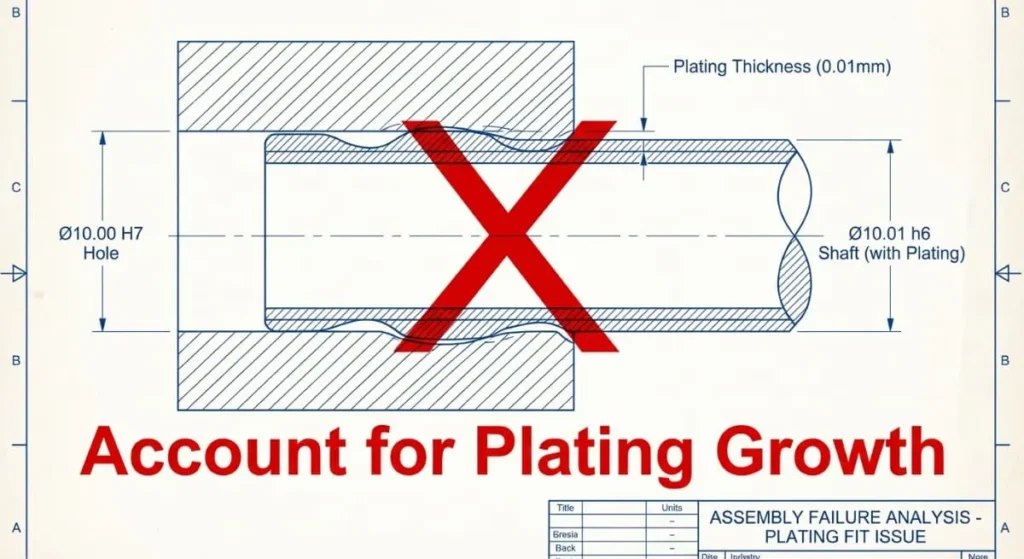
2. The Tolerance Stack-Up
Engineers often forget that this process adds material.
- Scenario: You machine a shaft to 10.00mm. You request 0.01mm Nickel plating.
- Result: The shaft is now 10.02mm (0.01mm per side). It will not fit in the hole.
- Solution: You must dial back your CNC machining targets to account for the electroplating growth.
Quick Selection Matrix
| Plating Type | Primary Function | Relative Cost | RoHS Risk? |
| Zinc (Clear) | Basic Rust Protection | $ (Low) | Low |
| Zinc (Yellow) | Durable Protection | $ (Low) | High (Hex Chrome) |
| Electroless Nickel | Precision & Uniformity | $$$ | Low |
| Hard Chrome | Heavy Wear Resistance | $$$ | Low |
| Gold | Connector Conductivity | $$$$$ | Low |
| Tin | Soldering | $ | Low |
Frequently Asked Questions
Q: Can you plate Aluminum?
A: Yes, but it is complex. Aluminum requires a “Zincate” strike layer before the final metal deposition. Not every shop can handle this.
Q: What is the difference between Anodizing and Plating?
A: Anodizing converts the surface into an oxide, while electroplating adds a layer of new metal. Anodizing is non-conductive; Plating is usually conductive.
Q: How do I measure layer thickness?
A: We use X-Ray Fluorescence (XRF) equipment to measure coating thickness non-destructively down to the micron.
Ready to finish your project?
Selecting the right finish is just as important as the machining itself. Contact CNMP for a comprehensive review of your materials and electroplating specifications.

